With over a decade of expertise in Singapore’s regulatory affairs, we’re dedicated to guiding you through the intricacies of the process.
- Planning to import or wholesale medical devices in Singapore? You must have an Importer and Wholesaler license.
- Certification: Obtain a Singapore Standard the Good Distribution Practice for Medical Devices (GDPMDS) SS620 certification from a certification body accredited by the Singapore Accreditation Council (SAC). This ensures the safety, quality, and performance of medical devices throughout the distribution process.
- Let us help you navigate the licensing process and ensure compliance with regulatory standards. Contact us today for a free consultation.

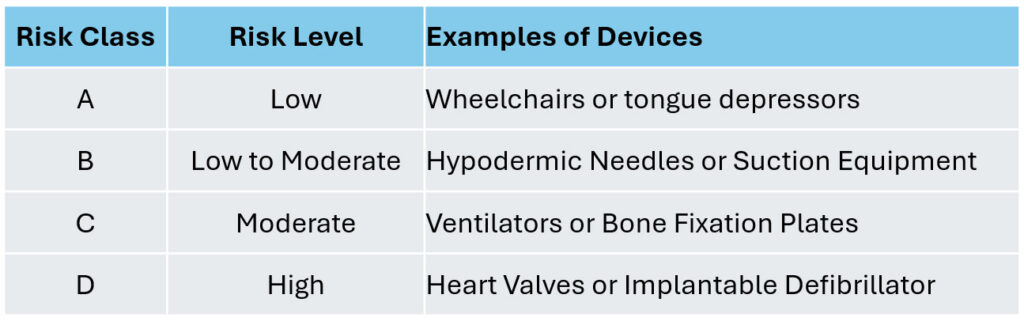
Specializing in Medical Devices Registration for all classes of Risks.

Get first access to our latest products, promotion & events.
